
Background:
Several clinical factors have been proposed to predict successful tyrosine kinase inhibitor (TKI) discontinuation for treatment-free remission (TFR), among which a longer duration of total TKI or deep molecular response (DMR) duration correlate with increased success of TFR. Although many guidelines have been proposed to safely select patients who would be candidates for TFR attempt, there is some discrepancy regarding which duration of DMR and/or Imatinib (IM) treatment optimally stratifies patients according to their probability of TFR success. These suggested DMR or IM treatment durations are often the result of a panel consensus, and the method of calculation of these thresholds as categorical values is not provided.
Thus, there is a practical need for an evidence-based determination of duration of IM therapy while awaiting TFR attempt to establish if the likelihood of successful TKI discontinuation reaches an optimal maximum after a certain duration of treatment and/or DMR. The present study attempted to define the optimal (i.e. shortest) duration of IM treatment or DMR that predicts TFR success at a specified level of confidence.
Patients and methods:
The Canadian TKI discontinuation study has enrolled 131 patients with the longest Imatinib treatment duration, at a median of 9 years. A Cox's proportional hazard ratio model was applied using molecular relapse-free survival (mRFS) as the endpoint. Continuous variables were initially tested using Cox's proportional hazard model and were converted into categorical variables according to the optimal cut-off values derived from the current analysis. We have evaluated six statistical parameters to determine the optimal cut-off of IM treatment duration and MR4 response duration for TFR prediction: 1) mRFS rate at 12 months between the groups, stratified according to the cut-off value, 2) proportion of patients divided by the cut-off value, 3) negative predictive value (NPV), 4) positive predictive value (PPV), 5) accuracy, and 6) the p-values as a measure of risk stratification power. The optimal cut-off was sought that met the joint criteria of a p-value ≤ 0.05, PPV≥60% and NPV≥60%.
Results:
Out of 131 patients enrolled, 123 patients completed a planned follow-up of 2.5 years. The mRFS rate was calculated as 56.8% (47.8-64.8%) at 12 months. One additional year of IM therapy increased the chance of TFR success by 5.5%, while one additional year of MR4 duration increased its likelihood by 5.1% by assessing the mRFS rates after 5 versus 9 years. The formula generated from this linear regression analysis is as follows: The probability of mRFS (%) = 0.05146 x (IM duration in year) + 0.08379; or 0.0555 x (MR4 duration in year) + 0.1844
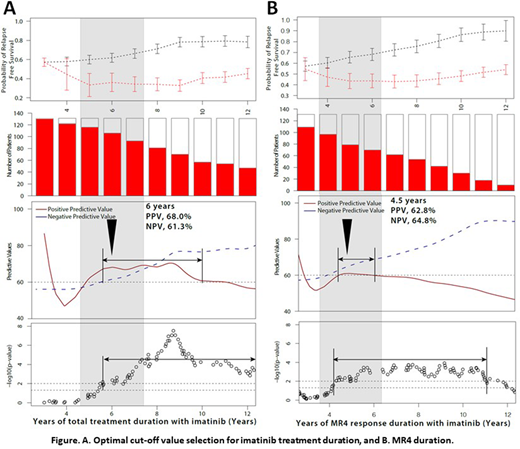
For example, as shown in Figure A, a patient with a total IM treatment duration of less than 6 years has a mRFS rate of 36.0% (n=25), implying a high risk of TFR failure, while those patients with IM duration of above 6 years showed a mRFS rate of 61.8% (n=106). PPV is defined as the probability of TFR failure in a subject at high risk for TFR failure at the proposed cut-off, while NPV is defined as the probability of TFR success in a subject at low risk for TFR failure (i.e. at low chance of TFR success) at the proposed cut-off. An ideal cut-off value should have both a high PPV (68%) and NPV (61.3%), thus defining 6 years as the optimal cut-off. The next parameter evaluated is the p-value of each cut-off value as a measure for risk stratification power; an acceptable p-value is ≤ 0.05. IM duration above 5.6 years and MR4 duration in the range of 4.2-11 years meet these criteria, respectively. Accordingly, the optimal cut-off value for IM treatment duration is ~ 6 years (Figure A), while that for MR4 duration is ~ 4.5 years (Figure B).
According to these cut-off values evaluated, patients treated with IM duration of 6 years or longer showed a superior mRFS rate at 12 months (61.8%) than those with less treatment (36.0%; p=0.01). Patients with MR4 duration of 4.5 years or longer showed a higher mRFS rate at 12 months (64.2%) than those with a shorter duration of deep molecular response (41.9%; p=0.003).
Conclusion:
In summary, we propose 6 years as the cut-off for IM duration with p-value=0.01, 68% PPV and 62% of NPV, while 4.5 years' cut-off value for MR4 duration is proposed with p-value=0.003, 63% of PPV and 61% of NPV. These results can be incorporated into clinical guidelines as optimal IM duration or MR4 duration for IM discontinuation to achieve successful TFR.
Bence-Bruckler:Merck: Membership on an entity's Board of Directors or advisory committees. Busque:Novartis: Honoraria; Pfizer: Honoraria; BMS: Honoraria. Delage:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Keating:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Hoffman La Roche: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Merck: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Consultancy; Servier: Membership on an entity's Board of Directors or advisory committees; Shire: Membership on an entity's Board of Directors or advisory committees; Taiho: Membership on an entity's Board of Directors or advisory committees. Lipton:Novartis: Consultancy, Research Funding; Bristol-Myers Squibb: Honoraria; Ariad: Consultancy, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding. Leber:Otsuka Pharmaceutical: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Treadwell: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda/Palladin: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Lundbeck: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal